electrolyte (salt) solution at room temperature. This is called charge inversion
phenomenon. Here, the "macroion" stands for an ion that is larger in radius
and charge content than ordinary ions like sodium Na or calcium Ca. People
who are familiar with the Debye screening theory in electrolyte liquid may have
difficulty in accepting this fact. Namely, the screening theory insists that the
test charge of positive sign be monotonically shielded by cloud of positive charges.
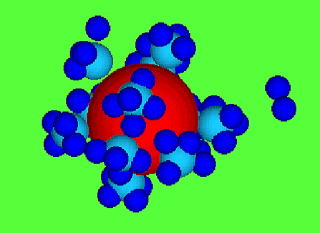
Giant charge inversion of a macroion (red) with trivalent counter-ions
(light blue) and monovalent co-ions (blue)
However, this strange phenomenon occurs when the electrostatic energy,
the source of structuralization, is larger than thermal energy that cause
diffusion, and also simultaneously when the gathering counterions are
multivalent charged like calcium Ca+2 or aluminum Al+3 ions.
The above condition corresponds to the strongly Coulomb coupled state
where ions are highly correlated, recognizing each others positions. The
means that the particle distribution is deviated from the Boltzmann
distribution which holds in ordinary thermal conditions. As previously
mentioned, this correlation causes the attraction force to exceed the repulsive
force, leading to structure formation in electrically neutral solvent (and
plasma). In fact, we get a Coulomb crystal at very low temperatures. The
figure above depicts a charge inverted macroion (red sphere), on which
trivalent counterions (light blue sphere) are electrostatically adsorbed.
The dark blue spheres are coions of monovalent charges which are some
distance apart from the macroion due to repulsion and condensed on the
topside of the counterions. As easily understood, the counterions must
have larger valence than the coions so that the counterion charges are not
cancelled by the coions.
Further we have shown that the polymer counterions (polyelectrolyte)
and the rod macroions are more favorable for charge inversion, especially
for a weakly charged macroion around the threshold surface charge density,
including the DNA (the figure and reference below).
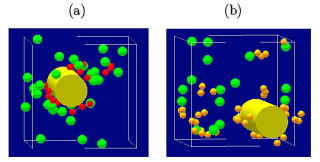
Charge inversion by polymer counterions consisting of (a) trivalent (red)
and (b) monovalent (yellow) monomers
References:
Review of ionic soft condensed matters, Solid State Physics (in April 2002).
Giant charge inversion of a macroion due to multivalent counterions and
monovalent coions, J.Chem.Phys.,115, 567-574 (2001).
The effects of asymmetric salt and a cylindrical macroion on charge inversion:
Electrophoresis by molecular dynamics simulations, Phys.Rev. E68, 061501 (2003)..
Electrophoresis of a rod macroion under polyelectrolyte salt:
Is DNA charge inverted ? J.Physics: Condensed Matter, 16, S21277-2134 (2004).
Giant charge inversion of a macroion due to multivalent counterions and
monovalent coions, J.Chem.Phys.,115, 567-574 (2001).
The effects of asymmetric salt and a cylindrical macroion on charge inversion:
Electrophoresis by molecular dynamics simulations, Phys.Rev. E68, 061501 (2003)..
Electrophoresis of a rod macroion under polyelectrolyte salt:
Is DNA charge inverted ? J.Physics: Condensed Matter, 16, S21277-2134 (2004).
.
.